First described in Drosophila and named after the "spiked" phenotype of the mutant fruit fly larva, the hedgehog (Hh) signaling pathway is highly conserved from flies to humans and regulates normal cell growth and differentiation in embryonic development. In adult organisms, the signaling pathway is largely inactive and limited to stem cell subpopulations to maintain tissue homeostasis. If imbalanced, the signaling pathway can lead to tumorigenesis and promote the spread of existing tumors (Reviewed in Doheny et al., 2020). A consistently activated Hh signaling pathway is found in a group of human cancers that together account for about 25% of all cancer deaths2. This has also led to the recognition of this signaling pathway as an important therapeutic target in the fight against malignant tumors.
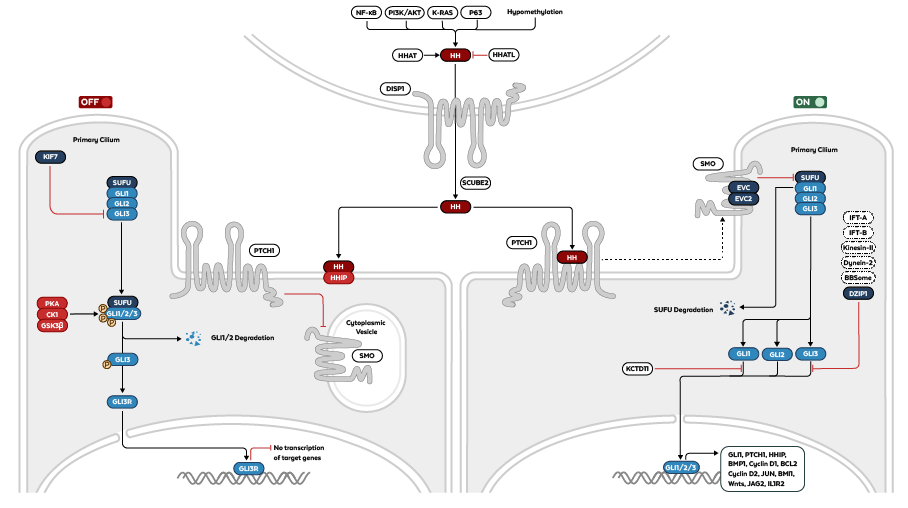
Figure: Mammalian hedgehog signaling pathway in its OFF (left) and ON (right) state.
Three homologs of the Hh protein are expressed in mammals. These include Sonic hedgehog (SHH), named after the video game character that helped SEGA gain success in the 1990s, indian hedgehog (IHH), and desert hedgehog (DHH). These proteins are generated from precursors through a multitude of processing steps including autocatalytic cleavage, ligation of cholesterol, and subsequent palmitoylation catalyzed by Hh acyl transferase (HHAT)3. Mature Hh is then secreted through cell surface protein dispatched-1 (DISP1) and binds to patched receptor (PTCH1) in target cells, lifting its inhibitory function on smoothened receptor (SMO). SMO subsequently activates the glioma-associated oncogene transcription factors (GLIs) that translocate to the nucleus and turn on the transcription of target genes.
In the off-state, inactive PTCH1 prevents the translocation of SMO to the cell surface. GLI proteins are then sequestered by suppressor of fused (SUFU) and phosphorylated by several kinases such as PKA, CK1, and GSK3β. Partial degradation of GLI proteins generates the repressor GLI3R which suppresses transcription of Hh target genes in the nucleus4. Rockland provides several antibodies to help study the hedgehog signaling pathway including highly specific polyclonals against GLI1, GLI2, GLI3, and SMO.
Hedgehog Signaling Antibodies
| Product | Clonality | Reactivity | Application |
| BMI1 Antibody | Polyclonal | Human, Mouse, Rat | WB, IF, IHC, ELISA |
| BMI1 Antibody | Polyclonal | Human | WB, IF, ELISA |
| Cyclin D1 Antibody | Polyclonal | Human | WB, ELISA |
| DISP1 Antibody | Polyclonal | Human, Mouse | WB, IF, IHC, ELISA |
| DISP2 Antibody | Polyclonal | Human, Mouse, Rat | WB, ELISA |
| DISP2 Antibody | Polyclonal | Human | IHC, ELISA |
| Gli1 Antibody | Polyclonal | Human, Mouse | WB, IF, IHC, ChIP, ELISA |
| Gli2 Antibody | Polyclonal | Mouse | WB, IF, IHC, ELISA |
| GLI2 Antibody | Polyclonal | Human, Rat | WB, IHC, ELISA |
| GLI3 Antibody | Polyclonal | Human | WB, IF, IHC, ELISA |
| GSK3 Beta phospho S9 Antibody | Polyclonal | Human | WB, IHC, ELISA |
| HHATL Antibody | Polyclonal | Human, Mouse, Rat | WB, IF, IHC, ELISA |
| SCF Antibody | Polyclonal | Human, Mouse, Rat | WB, IF, IHC, ELISA |
| Smoothened Antibody | Polyclonal | Human | WB, IHC, ELISA |
| Wnt1 Antibody | Monoclonal | Mouse | WB |
| Wnt1 Antibody | Polyclonal | Human, Mouse | WB, ELISA |
| Wnt5A Antibody | Polyclonal | Human, Mouse, Rat | WB, IHC |
| Wnt10a Antibody | Polyclonal | Human, Mouse, Rat | WB, IF, IHC, ELISA |
| Wnt10B Antibody | Polyclonal | Human, Mouse, Rat | WB, IHC, ELISA |
References
- Doheny, D., Manore, S. G., Wong, G. L., & Lo, H. W. (2020). Hedgehog Signaling and Truncated GLI1 in Cancer. Cells, 9(9), 2114.
- Lum, L., & Beachy, P. A. (2004). The Hedgehog response network: sensors, switches, and routers. Science (New York, N.Y.), 304(5678), 1755–1759.
- Kandel, N., & Wang, C. (2022). Hedgehog Autoprocessing: From Structural Mechanisms to Drug Discovery. Frontiers in molecular biosciences, 9, 900560.
- Gupta, S., Takebe, N., & Lorusso, P. (2010). Targeting the Hedgehog pathway in cancer. Therapeutic advances in medical oncology, 2(4), 237–250.